
Its frequency is thus the Lyman-alpha hydrogen frequency, increased by a factor of ( Z − 1) 2. Because the 2p electron is not screened by any other electrons in the atom from the nucleus, the nuclear charge is diminished only by the single remaining 1s electron, causing the system to be effectively a hydrogenic atom, but with a diminished nuclear charge Z − 1. This is analogous to the Lyman-alpha line transition for hydrogen, and has the same frequency factor.
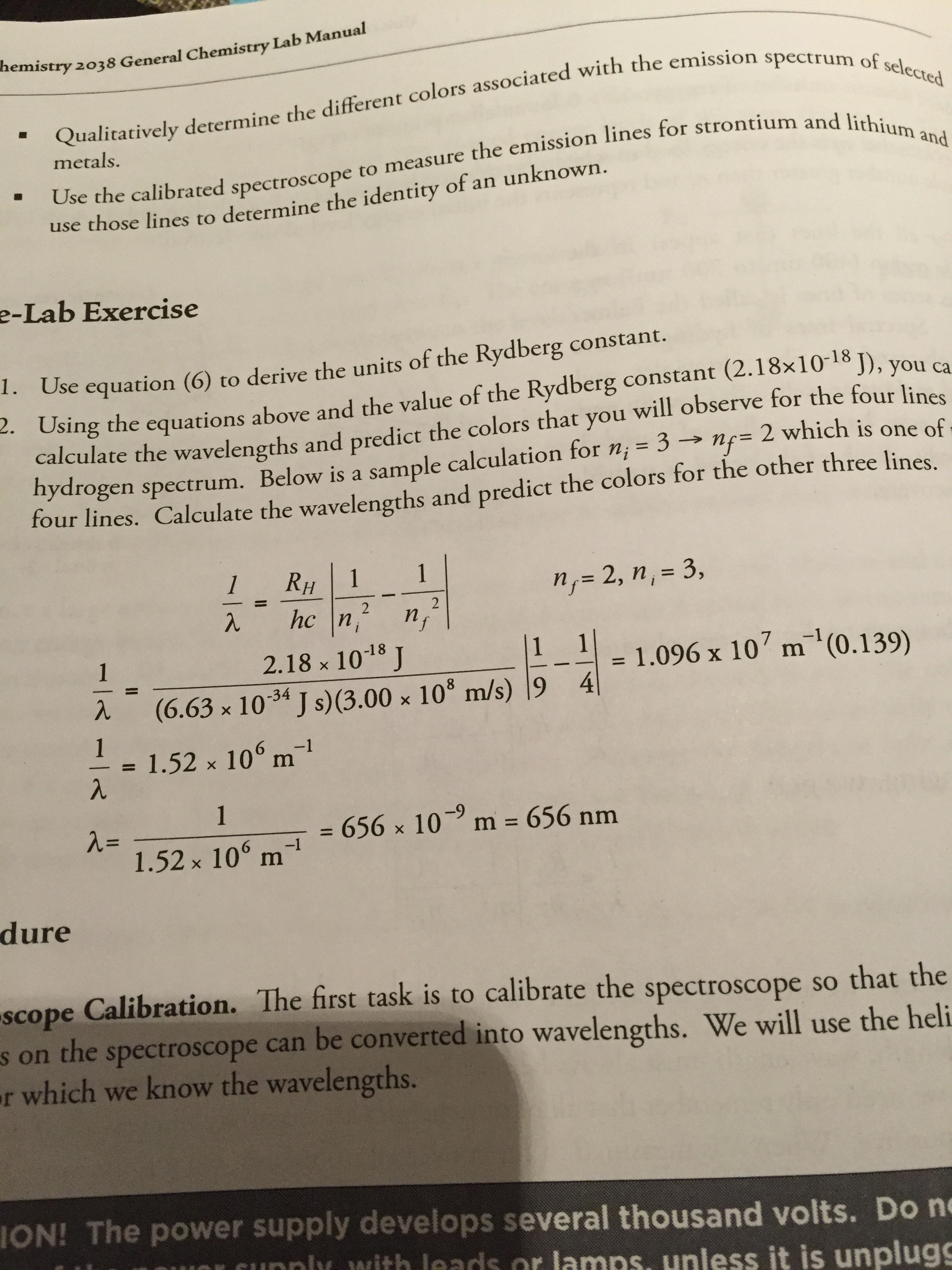
Examples would include He +, Li 2+, Be 3+ etc., where no other electrons exist in the atom.īut the Rydberg formula also provides correct wavelengths for distant electrons, where the effective nuclear charge can be estimated as the same as that for hydrogen, since all but one of the nuclear charges have been screened by other electrons, and the core of the atom has an effective positive charge of +1.įinally, with certain modifications (replacement of Z by Z − 1, and use of the integers 1 and 2 for the ns to give a numerical value of 3⁄ 4 for the difference of their inverse squares), the Rydberg formula provides correct values in the special case of K-alpha lines, since the transition in question is the K-alpha transition of the electron from the 1s orbital to the 2p orbital.

atoms with only one electron being affected by an effective nuclear charge (which is easily estimated). This formula can be directly applied only to hydrogen-like, also called hydrogenic atoms of chemical elements, i.e. Finding that the resulting curves were similarly shaped, he sought a single function which could generate all of them, when appropriate constants were inserted.įirst he tried the formula: n = n 0 − C 0 m + m ′ is the principal quantum number of the higher energy level for the atomic electron transition.
Rydberg equation calculator series#
He plotted the wavenumbers ( n) of successive lines in each series against consecutive integers which represented the order of the lines in that particular series. He noticed that lines came in series and he found that he could simplify his calculations using the wavenumber (the number of waves occupying the unit length, equal to 1/ λ, the inverse of the wavelength) as his unit of measurement. In 1880, Rydberg worked on a formula describing the relation between the wavelengths in spectral lines of alkali metals.


 0 kommentar(er)
0 kommentar(er)
